Case Studies: Research & Development
Co-Creating a Pill
Project brief
Situation
A company is developing an oral pill for the treatment of a rare disease. Compared to the current standard of care, also a pill, this drug contains a new active ingredient. The new drug has been tested in a first phase II trial in patients, with good results on both efficacy and safety. Now, the company is preparing a pivotal trial. At this point in time, a patient advocate contacts the chief scientific officer (CSO) of the company. She has a number of questions from the patient community she hopes to get answers to. The CSO immediately invites her for a meeting at the company’s facilities.
Objective
The patient advocate wants to inform the patient organization’s members about the potential new therapeutic option, with information directly obtained from the developer.
Procedure
Approach
- In preparation of the interview, the patient organization drafts an interview guide covering questions, expectations and wishes regarding the new treatment option.
- Next, they reach out to over 3,000 patient forum members worldwide, via various Facebook forums, asking them to post any questions they have about the new drug.
- Based on the feedback they add and adapt the interview guide.
- Approximately 20% of the feedback from patient forum members is related to their difficulties swallowing the current pill.
- At the meeting, the CSO and the international project leader (Lead) of the development program give a presentation about their new treatment and the status of development.
- The first question of the patient advocate is whether the new drug is available in a liquid form. She explains the swallowing problems. Both the CSO and the Lead admit they were not aware of the issue. They were not planning on developing a liquid form.
- The patient advocate also asked whether the new drug treatment would be as bitter as the current pill, since many patients have a hard time dealing with the taste. The Lead explains that this will not change with the new drug, as it is a characteristic of the active substance. Masking the taste would probably be possible, but he frankly admits this is currently not high on the priority list of the company.
- While discussing the potential improvements of the new pill, the three participants get very excited about the prospect of a possible future collaboration.
- They end the meeting with an agreed follow-up meeting soon, including other people from both organizations.
The long list for a draft agenda looks as follows:- What are current issues with the existing treatment?
- What would it take to reduce the size of the pill and improve the taste?
- What would it take to develop a liquid form?
- What can be done to develop a form for children soon?
- Could we collaborate on the design of the pivotal trial to come?
- Could we collaborate on setting up an international patient registry?
Evaluation
What went well what can be improved?
The openness of both sides to listen and learn from each other in an honest and respectful way are a good example in this case study. It is however thanks to the proactive approach of the patient advocate that both parties get into discussion. Had the company reached out much earlier in research and development, more could have been achieved at an earlier stage. Perhaps the administration form could have been suited to the patient’s needs right from the start?
Considerations and recommendations
Achieving the goal of shaping the best possible solution for patients in R&D requires involvement of people intended to benefit from that solution. They don’t replace any other stakeholder, not even health care practitioners, as they can add valuable insights to the process across the value chain no other stakeholder can provide. This also holds for instance in regard of packaging design (see also case study "Co-Creation: Solving Issues of Patients with the Product Packaging").
Many patient organizations and individual patients are very keen to contribute and open for direct collaboration with the industry – especially in R&D. Subject to the capabilities of the respective patient community in a certain therapeutic space, your company may soon identify many more valuable opportunities for collaboration, going way beyond the originally defined subject of discussion. The graph below, provided by CTTI, for collaboration on clinical trials can be used as an orientation to shape patient engagement in several stages.
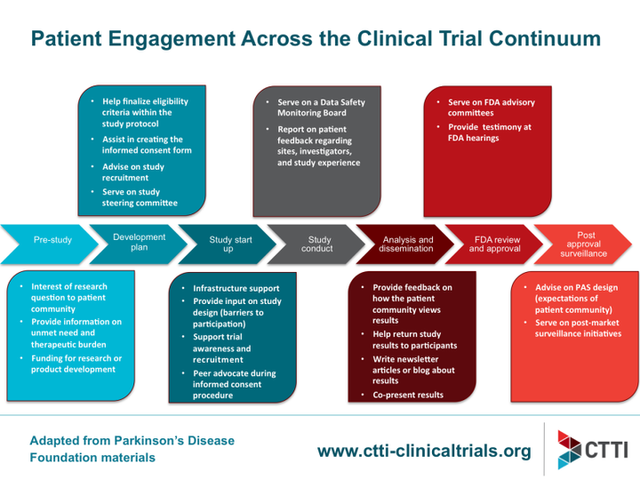
CTTI 2018: Patient Engagement Accross the Clinical Trial Continuum (02/05/2018)
Talk to patients early and systematically! To get started, you may want to check the case study "First Time Contact to Patients from the Industry's View".
Checklist
Mapping the Patient Landscape
Following a general understanding of the patient journey and unmet needs and before systematically interacting with patients and KOLs in a specific therapeutic area a company should have an understanding of the stakeholder field. The checklist below can help you obtain such an understanding, by:

Source: This checklist has been developed by admedicum® Business for Patients GmbH.
Download
Help us improve this guidebook
Submit Feedback
Tool
Considerations for Implementing Expert Patient/Patient Group Input
Recommended Contributors :
- Program leaders
- Patient liaisons
- Sponsor representatives
- Clinical investigators
- Research team
- Trial site staff
- IRB
- Expert patient(s)/Patient Group representatives
Communicating with Patients throughout the Program
- How does the phase of drug/biologic/device development process covered by this program impact communication with patients?
- What translation and/or cultural adaptions?
- Wat language will be used to communicate with and about the patients?
- Are research questions and procedures culturally sensitive and appropriate?
- How will patients be referred to (e.g. “subject” vs. “patient” vs. “participant”)?
- What is the communication plan for patients throughout the program?
- Message content
- Audience
- Messenger
- Delivery mechanisms
- Timing
- Feedback mechanisms
- What feedback mechanisms and processes are in place for the patients to comment on sites, investigators, and the study participant experience?
- What role will social media play in the communications?
- How is social media defined?
- What restrictions should there be, if any?
- How can social media be used to advantage (e.g. for trial recruitment, to educate patients)?
- What limits should be placed on use of social media, if any? Why?
- How will those limits be communicated and enforced?
- What methods will be used to interact with patients and other stakeholders?
- Focus groups
- Interviews
- Surveys
- Inclusion on advisory councils
- Inclusion in meetings with researchers
- What data/information can and will be shared with the patients and when?
- Aggregate (de-identified)
- Patient-specific
- What are the restrictions (propieratry and regulatory) constraining the release of data?
- How do we ensure that this information is shared in patient-friendly language? How will that be determined/monitored?
Additional resources
Communication Handbook for Clinical Trials.
Guidance for Biomedical HIV Prevention Trials, p 37-38: “Stakeholder education plan.”
Checklist
Preparing a collaboration
Defining the interaction
Patients, patient representatives and industry should take responsibility to ensure interactions are meaningful by clearly defined processes and actions, progressed to timelines. In addition, all participants should be prepared for the interaction.
Prior to each interaction, agree mutually on (where applicable):
- The objective of project involving patients and/or areas of common interest to establish agreed structured interaction, providing all parties with necessary protection with regards to independence, privacy, confidentiality and expectations (see section 11. written agreement)
- The type of input and mandate of the involved person
- The tools and methods of interaction, e.g. types and frequency of meetings, ground rules, conflict resolution, evaluation
- Desired patient / patient partner organisation to foster long-term working partnerships, with independence ensured (in scope)
- The profile of the type of patient/s or patient representatives/s to be involved and their number
- How activity outputs will be used and ownership of outputs
- How and when the patient/s involved will be informed of outcomes
- Contractual terms and conditions including consent and compensation (see section 11, written agreement).
- Other elements according to the specific project
Source
Checklist
Preparing a collaboration
The four key principles for collaboration:
1. Clarity of Purpose
Each party should be clear about the reason for and the planned outcome of the collaboration – and the ultimate benefit for patients
2. Integrity
Each party should act and be seen to act honestly and with integrity at all times
3. Independence
Each party should maintain their independence
4. Transparency
Each party should be open and honest about the purpose of the collaboration and be able to account publicly for the associated activities and any exchanges of funding
Using this guide: a checklist
- Has there been a frank discussion about the purpose and expected benefits of the collaboration, and any risks, addressing all the issues in this guide?
- Are the objectives and planned outcomes of the collaboration specified?
- Are the roles of each partner and reporting mechanisms specified?
- Has a written agreement or contract been put in place, which sets out how each party will adhere to the four key principles?
- Is there a named senior individual accountable for managing and maintaining the relationship and monitoring adherence to the four key principles?
- Is information about the collaboration published on the company and charity websites?
- Can each party confidently explain the collaboration in public?
Source: National Voices, The Association of the British Pharmaceutical Industry (ABPI) (2015): Working
Checklist
Patient identification/interaction
Patient identification/interaction
There are many ways to identify patients to be involved in an interaction. The main routs are through:
- existing patient organisations
- EUPATI or similar project
- advertising opportunities for patient participation
- existing relationships with healthcare providers, hospitals and researchers and other agencies
- unsolicited requests previously made by interested parties
- existing advisory boards / groups (e.g. EFPIA Think Tank, Patients and Consumers Working Party at the EMA)
- this party agencies
Source: European Patients’Academy on Therapeutic Innovation (EUPATI) (2016): Guidance for patient involvement for industry-led medicines R&D. (12/06/17)
Tool
List of useful conferences
Conferences with and for patients
Rare diseases conferences with and for patients
The Global Orphand Drug Conference and Expo
Indication specific conferences with and for patients
Tool
Defining role patients
Although you may not have selected the expert patients or patient groups (EP/PGs) yet, outlining their roles and responsibilities at this stage helps to define your needs. Keep in mind that EP/PG roles may vary at different of the program or may evolve in response to new requirements. Once selected, discuss the roles with your EP/PGs to clarify what they can contribute based on their unique expertise and experience and avoid misunderstandings at the outset, e.g., if they’re expecting to have a partnership role but you’ve designed reactor role (see Types of Patient Roles chart below).
| Patient Role | Examples | Engagement Level |
| Partnership role |
| High |
| Advisor role |
| Moderate |
| Reactor role |
| Low |
| Trial or study participant |
| None |
Source: DIA (2017): Considerations Guide to Implementing Patient-Centric Initiatives in Health Care Product
Development. (02/06/17)